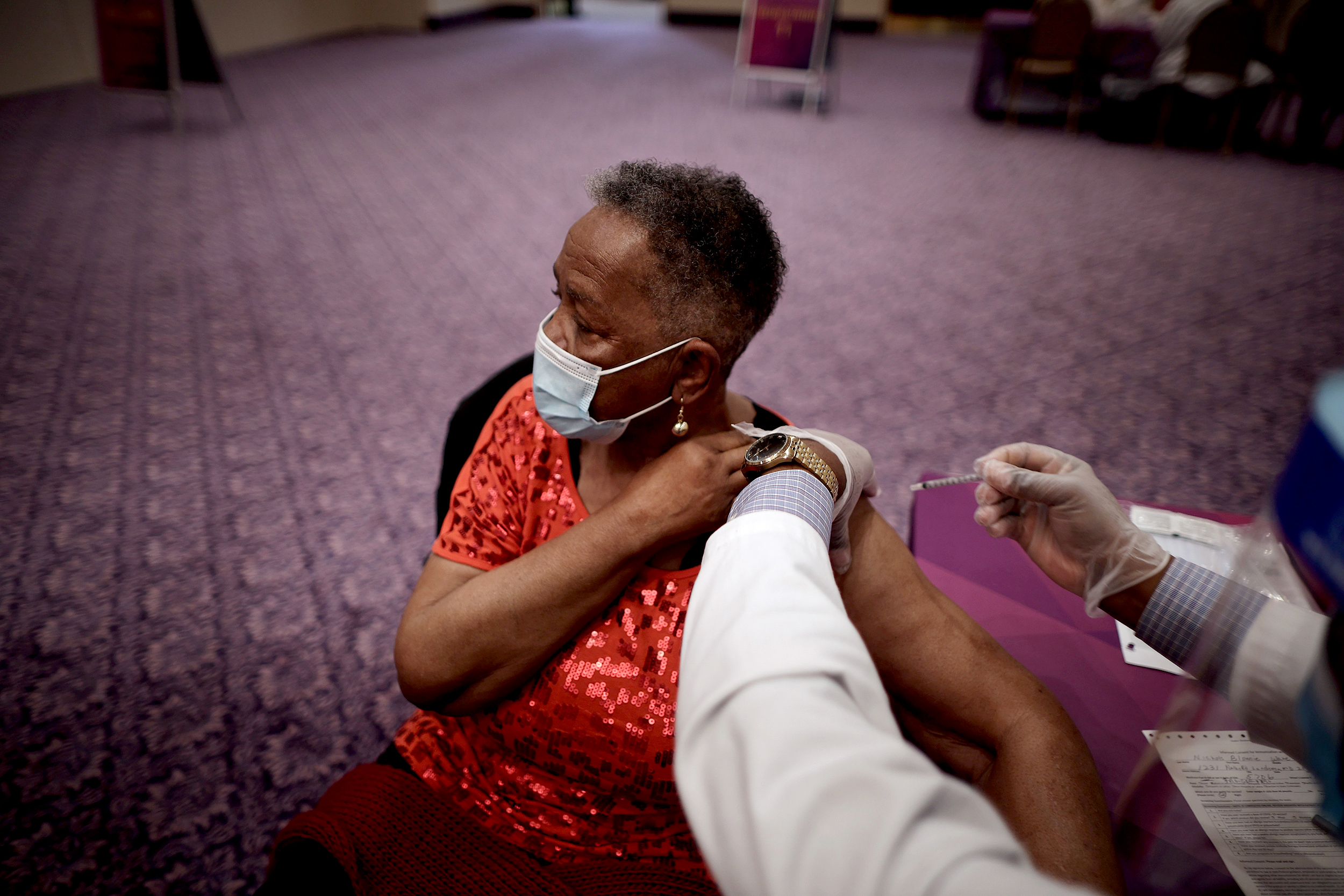
The Covid-19 vaccine developed by Pfizer and BioNTech has been recommended for an emergency use authorization by the FDA’s vaccine advisors. | Angela Weiss/AFP via Getty Images
The first doses of the Pfizer/BioNTech vaccine are expected to ship soon.
An advisory committee to the Food and Drug Administration has voted 17 to 4, with 1 abstention, to recommend emergency use authorization (EUA) to the first vaccine for Covid-19 in the United States. In response, FDA officials said Friday the agency will accept the committee’s recommendation and work to issue the EUA as soon as possible.
The highly anticipated vote means health workers facing high exposure to the disease and residents of long-term care facilities could start receiving the first doses of the two-dose vaccine, developed by Pfizer and BioNTech, within days. The first 6.4 million doses could ship as soon as Friday