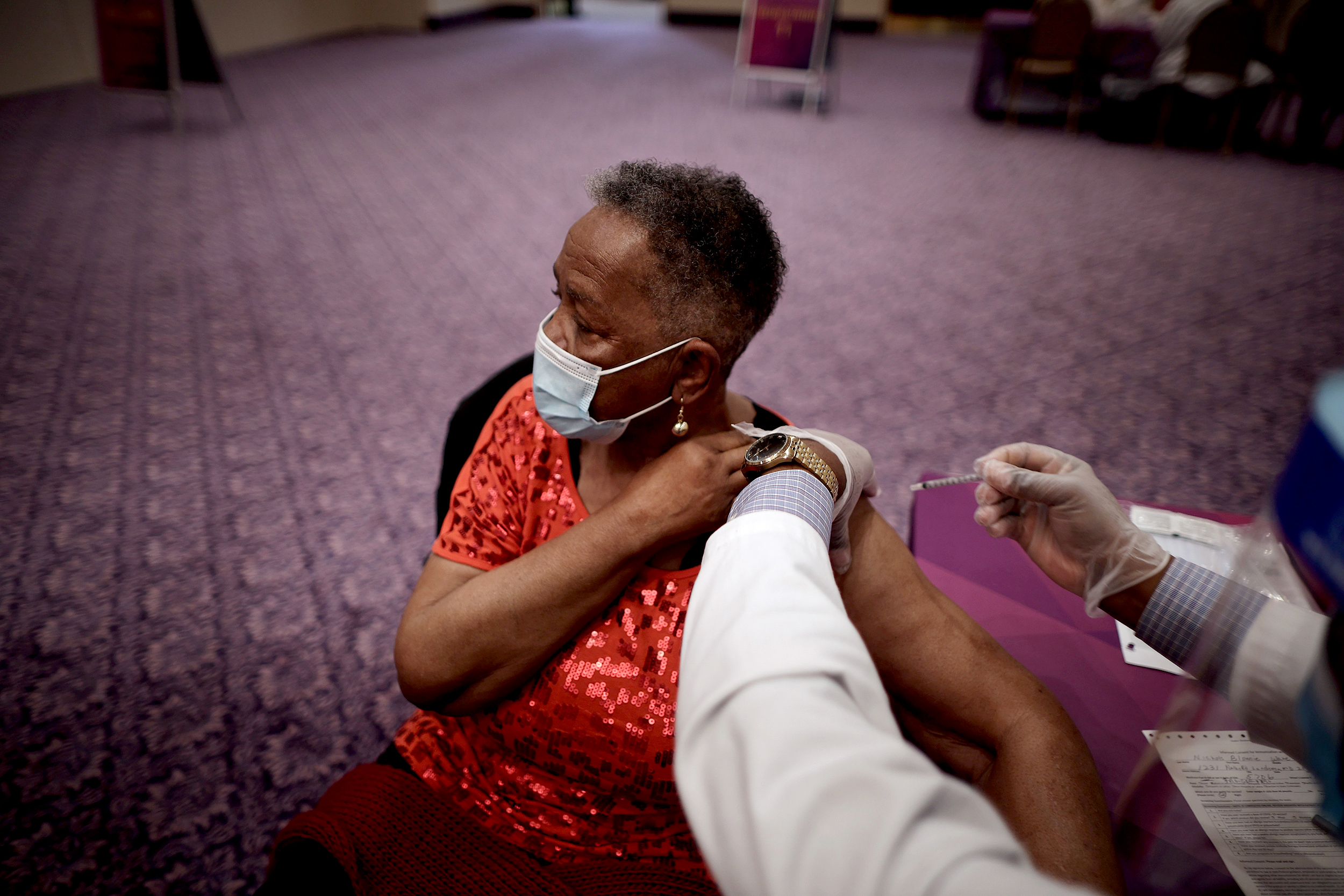
With two Covid-19 vaccines being distributed, researchers are debating when and how to vaccinate clinical trial participants who received a placebo. | Leila Macor/AFP via Getty Images
Thousands of people in clinical trials got the placebo instead of the vaccine. When should they get the shots?
With supply of the two authorized vaccines for Covid-19 still limited, there’s been a lot of chatter around how exactly to distribute the initial doses in the US. But the prospect of vaccinating one group in particular has proven to be especially vexing for scientists and health officials: people participating in phase 3 clinical trials for these vaccines.
The ongoing phase 3 trial for the Pfizer/BioNTech vaccine includes 44,000 people, and 22,000 received the placebo. For the Moderna vaccine trial, half of the 30,000 participants got it.
These trials demonstrated that both of